AKKUS
A battery is a device that converts chemical energy directly to electrical energy.It consists of a number of voltaic cells; each voltaic cell consists of twoconnected in series by a conductive electrolyte containing anions and cations. One half-cell includes electrolyte and the electrode to which(negatively charged ions) migrate, i.e., the anode or negative electrode; the other half-cell includes electrolyte and the electrode to which (positively charged ions) migrate, i.e., the cathode or positive electrode. In thereaction that powers the battery, reduction (addition of electrons) occurs to cations at the cathode, while oxidation (removal of electrons) occurs to anions at the anode.The electrodes do not touch each other but are electrically connected by the . Many cells use two half-cells with different electrolytes. In that case each half-cell is enclosed in a container, and a separator that is porous to ions but not the bulk of the electrolytes prevents mixing.
Each half cell has an electromotive force (or emf), determined by its ability to drive electric current from the interior to the exterior of the cell. The net emf of the cell is the difference between the emfs of its half-cells, as first recognized by Volta. Therefore, if the electrodes have emfs  and
and  , then the net emf is
, then the net emf is 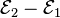 ; in other words, the net emf is the difference between theof the
; in other words, the net emf is the difference between theof the
The electrical driving force or  across the terminals of a cell is known as the terminal voltage (difference) and is measured in The terminal voltage of a cell that is neither charging nor discharging is called the and equals the emf of the cell. Because of internal resistance, the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage. An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of
across the terminals of a cell is known as the terminal voltage (difference) and is measured in The terminal voltage of a cell that is neither charging nor discharging is called the and equals the emf of the cell. Because of internal resistance, the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage. An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of  until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one then on complete discharge it would perform 1.5 of work.In actual cells, the internal resistance increases under discharge,and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.
until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one then on complete discharge it would perform 1.5 of work.In actual cells, the internal resistance increases under discharge,and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.
As stated above, the voltage developed across a cell's terminals depends on the energy release of the chemical reactions of its electrodes and electrolyte. Alkaline andcells have different chemistries but approximately the same emf of 1.5 volts; likewise and cells have different chemistries, but approximately the same emf of 1.2 volts.On the other hand the high electrochemical potential changes in the reactions ofcompounds give lithium cells emfs of 3 volts or more.